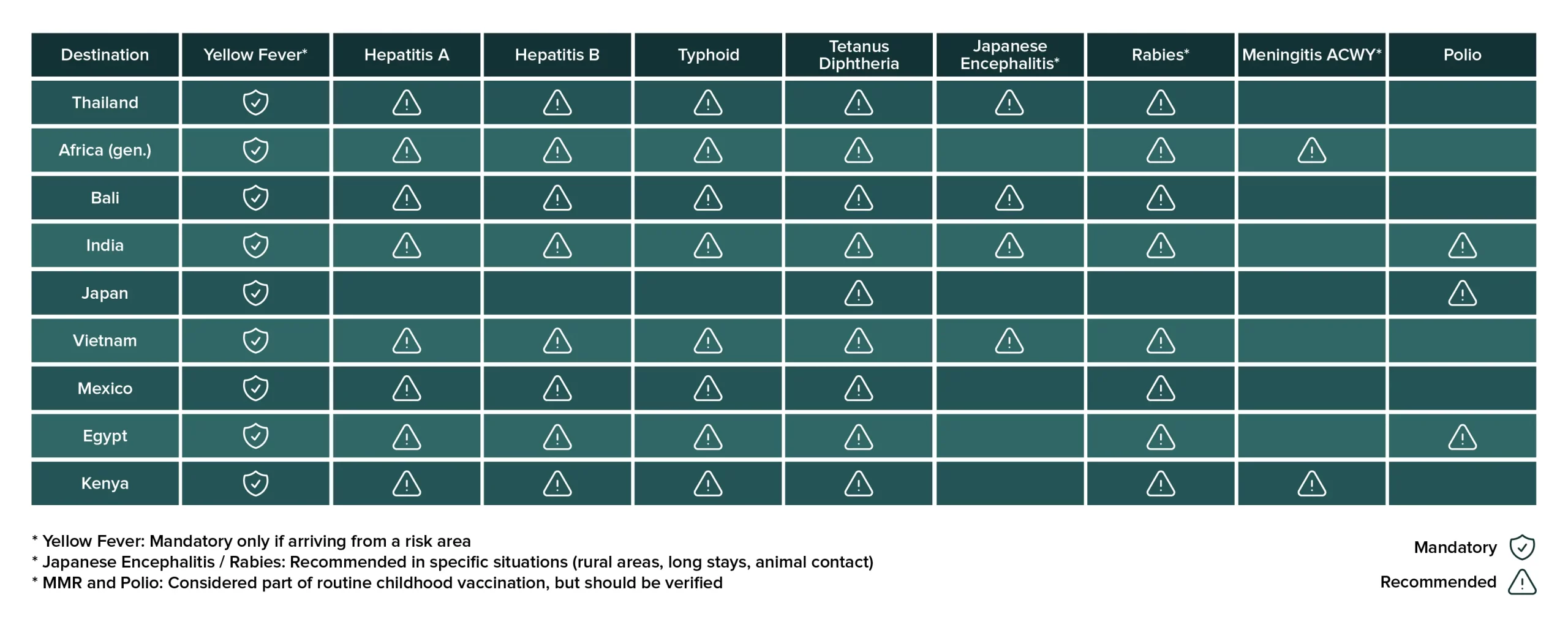
Travelling well protected is an essential part of planning any adventure. When organizing a trip to exotic destinations or countries with health risks, questions naturally arise: What vaccines are mandatory to enter certain countries? Which ones are recommended to protect my health during the trip? Below, we offer a complete vaccination guide for travellers, focusing on some of the most searched-for destinations such as Thailand, India, Japan, African countries, Vietnam, Mexico, Egypt, Kenya and Bali. We will explore why certain vaccines are required, the difference between mandatory and recommended vaccines, and explain in simple terms how the most relevant ones work (yellow fever, hepatitis A and B, typhoid fever, Japanese encephalitis, etc.).
Why do some countries require vaccinations for travellers?
Several countries impose vaccination requirements on travellers as a public health and border control measure. Yellow fever is the classic example: numerous countries in Africa and Latin America require an international certificate of vaccination against yellow fever from travellers coming from areas where the virus is endemic. This is done under the International Health Regulations, with the aim of preventing the import and spread of dangerous diseases within their territory. In practice, if you have been in a country with yellow fever (or even in airport transit for more than 12 hours) before entering, for example, Thailand, India, Egypt, or South Africa, authorities may request the WHO’s “yellow card” certifying your vaccination. Otherwise, you may be denied entry or quarantined for up to 6 days (as is the case with India).
In short, mandatory vaccines are usually few, but important. The yellow fever vaccine is the most commonly required internationally, due to the severity of the disease and the presence of vectors (mosquitoes) in tropical regions. Saudi Arabia also requires the meningococcal vaccine for Hajj pilgrims, and some countries have requested polio vaccination certificates from travellers coming from places where polio remains endemic or where outbreaks have occurred. During the COVID-19 pandemic, many destinations required vaccination or testing, although by May 2025, almost no country requires COVID vaccination for tourists.
As for recommended vaccines, these are not legally required for entry, but are advised to protect the traveller’s health given the risk of contracting certain diseases at the destination. For example, diseases transmitted through water or food (such as hepatitis A or typhoid fever), mosquito bites (such as dengue, malaria, Japanese encephalitis), contact with animals (such as rabies), or more universal infections that may have higher incidence in some regions (such as hepatitis B, tetanus, measles, etc.). Health authorities (WHO, CDC, national ministries of health, etc.) recommend visiting an International Vaccination Centre 4–6 weeks before your trip to assess your particular case. There, they will review your vaccination record and indicate which vaccines you should receive based on your destination, itinerary, and planned activities. Below, we’ll explore specific recommendations for some popular destinations.
What vaccinations do I need to travel to Thailand?
Thailand does not require any mandatory vaccines for travellers arriving directly from Europe or other regions without yellow fever. The only “mandatory” vaccine is for yellow fever, and only if you are coming from a country where the disease is endemic (such as certain parts of South America or Africa). Otherwise, no vaccinations are required to enter the country.
However, health authorities do recommend several vaccines for travel to Thailand, given the country's epidemiological profile. According to the Embassy of Spain in Bangkok, it is advisable to be vaccinated against hepatitis A and B, tetanus-diphtheria, Japanese encephalitis, and rabies, among others. Hepatitis A is a classic travel-related disease, transmitted through contaminated food and water; in fact, the CDC recommends it for all unvaccinated travellers visiting Thailand. Typhoid fever (Salmonella typhi) is also recommended for most travellers venturing beyond highly touristic circuits, especially those visiting rural areas or eating at local food stalls. The typhoid vaccine can be administered orally or by injection. While it doesn’t offer 100% protection, it provides useful partial immunity, but it doesn’t replace caution with food and water.
Thailand, like other Southeast Asian countries, has cases of Japanese encephalitis, a mosquito-borne virus found in rural areas and rice fields. This vaccine is not needed for all tourists but is considered for those spending extended time in rural areas, trekking through remote villages, or staying without mosquito nets. It is not generally recommended for short stays in cities or typical beach destinations (the risk in places like Bangkok or Phuket is virtually nil).
Lastly, rabies is present in street dogs and wildlife in Thailand. While the rabies pre-exposure vaccine is not mandatory, it is advised for travellers heading to rural areas, nature parks, or who expect close contact with animals (e.g., veterinarians, volunteers, children who may play with animals). If unvaccinated, it's crucial to know that in the event of a bite or scratch in Thailand, you must seek immediate medical attention to begin post-exposure prophylaxis (immunoglobulin and vaccines). The good news is that this treatment is widely available in most Thai cities.
In summary, the recommended vaccines for travel to Thailand are: Hepatitis A, Hepatitis B, Typhoid, Tetanus-diphtheria (up to date with Td or Tdap), Rabies (for at-risk travellers), and Japanese encephalitis (only for prolonged rural itineraries). Of course, all routine vaccinations should be current (MMR for measles, mumps, and rubella; seasonal flu, etc.), as outbreaks of preventable diseases can occur anywhere in the world when populations are not adequately vaccinated.

What vaccinations are recommended for travel to Africa?
Speaking about “Africa” in general is complex, as the continent includes dozens of countries with very different public health realities. However, most travellers to sub-Saharan Africa (safaris in Kenya/Tanzania, routes through South Africa, volunteering in West Africa, etc.) share some common vaccination recommendations.
In Africa, the almost universal mandatory vaccine is yellow fever. Many African countries require proof of yellow fever vaccination from all travellers over 1 year of age arriving from an area with risk of transmission. In fact, in many nations within tropical Africa, the yellow fever vaccine is not only an entry requirement but also a strong health recommendation for anyone visiting endemic regions. In other words, even if you are flying from Spain, if you're travelling to countries where yellow fever is present (such as Senegal, Nigeria, Kenya, etc.), it is advisable to get vaccinated for your own protection. Yellow fever is transmitted by mosquitoes in jungle areas and can be fatal; the vaccine is highly effective (nearly 100%), and a single dose provides lifelong immunity. It should be administered at least 10 days before travelling for the certificate to be valid internationally.
In addition to yellow fever, the following vaccines are commonly recommended for African itineraries:
- Hepatitis A: Large areas of Africa have poor sanitation, which means a high risk of hepatitis A for unvaccinated travellers. This is a very safe inactivated vaccine that is recommended for virtually any traveller to Africa who hasn't had the disease or been previously vaccinated.
- Typhoid fever: Similarly, typhoid fever is endemic in many African countries. The WHO notes that the overall risk for international travellers is generally low, except in developing areas of sub-Saharan Africa, where it increases significantly. Therefore, vaccination (oral or injectable) is advisable if you're going to interact with the local population, eat at street stalls, or visit rural areas.
- Hepatitis B: Hepatitis B is transmitted through blood and bodily fluids (sexual contact, medical procedures, tattoos, etc.) and has a high prevalence in Africa. Many younger travellers were vaccinated during childhood, but if that’s not your case, you should get vaccinated before a long trip to Africa. It’s a series of three doses that provides protection against a serious chronic liver disease.
- Meningococcal meningitis: In the “meningitis belt” of Africa (the Sahel region from Senegal to Ethiopia), seasonal outbreaks of meningitis occur. Countries in West Africa during the dry season may recommend the quadrivalent ACWY vaccine against Neisseria meningitidis. It's usually not necessary for Southern Africa or typical safari trips, unless you're planning to live in local communities in high-risk zones.
- Rabies: Africa reports thousands of rabies cases every year, mostly due to stray dogs. The pre-exposure rabies vaccine is advised for adventurous travellers planning to camp, hike, bike, or work with animals in Africa—especially in remote areas with limited access to healthcare. If not vaccinated, the traveller must take extra precautions to avoid animal contact and be prepared to act immediately in case of a bite.
- Tetanus-diphtheria: It's essential to be up to date with this vaccine (a booster every 10 years), as any wound in rural, unhygienic environments can expose you to the tetanus bacillus. It’s part of the routine vaccination schedule, but its importance before travelling is worth reinforcing.
- Polio: Although Africa was declared free of wild polio in 2020, outbreaks of vaccine-derived poliovirus still occur in some countries. The WHO recommends that travellers staying long-term in countries with polio (e.g., Nigeria, the Democratic Republic of the Congo) receive a booster dose if they haven’t had one recently. In general, make sure your childhood polio vaccination is complete; an extra adult booster is advisable if you're visiting countries that have recently reported cases.
Lastly, it's important to remember that malaria is endemic in many parts of sub-Saharan Africa. There is currently no vaccine for tourists (except for pilot programs in local children), so antimalarial prophylaxis (preventive medication like mefloquine, doxycycline, or atovaquone/proguanil depending on the region) and mosquito protection are essential. Consult your doctor to determine the appropriate antimalarial medication for your destination.

What vaccinations are recommended for travel to Bali?
Bali is an interesting case: it’s a popular destination in Indonesia, a tropical country with some endemic diseases, yet Bali itself is highly touristic. Indonesia does not require any mandatory vaccines for tourists in general, except again for the yellow fever vaccine if you’re coming from a country where the disease is present.
For Bali, the recommended vaccines are similar to those for other Southeast Asian destinations: Hepatitis A, Hepatitis B, Typhoid, Tetanus-diphtheria, and depending on your activities, Rabies and Japanese Encephalitis.
- Hepatitis A: Indonesia has areas with poor sanitation outside resort zones, so it’s advisable to be vaccinated.
- Typhoid fever: Recommended if you plan to eat outside very touristic areas or travel to less developed islands. Even in Bali, eating at local warungs carries some risk of typhoid, so the vaccine is recommended for long stays or backpackers.
- Hepatitis B: Recommended if you’re not already vaccinated, especially for younger travellers who may require medical care there, or plan to get tattoos, piercings, or engage in intimate contact.
- Japanese Encephalitis: Bali itself is not considered a high-risk zone for Japanese encephalitis compared to rural areas of Java or Lombok, but the disease is present in Indonesia (especially in rural regions with rice paddies and pigs). The vaccine may be considered if you plan to spend extended time in rural areas of Bali or visit other rural islands for more than a month, especially during the rainy season. For the typical 1–2 week tourist trip to beaches and temples, the vaccine is usually not necessary (mosquito protection is sufficient).
- Rabies: It’s important to note that Bali has had rabies outbreaks in stray dogs in recent years. In fact, it has become endemic on the island since the late 2000s. If your plans include surfing, hiking, or visiting villages with stray dogs, getting vaccinated against rabies may be worth it. At the very least, be informed and avoid approaching dogs or monkeys (Bali has many monkeys, for example in the Ubud Sacred Monkey Forest, which sometimes bite). Knowing that hospitals in Bali offer post-exposure rabies treatment is helpful, but there may be delays in rural areas. So consider the vaccine if you expect animal contact.
- Tetanus-diphtheria: Make sure your Td booster is up to date. Riding a motorbike in Bali is common, and a wound on a rural road where tetanus spores may be present is a real risk, better to be protected with a vaccine (which you likely already have).

What vaccinations do I need to take for my trip to India?
India does not impose mandatory vaccinations for international tourists, except for yellow fever for those arriving from countries where it is endemic. One important note: the Indian government does require travellers from countries with endemic poliomyelitis (currently Pakistan and Afghanistan) to present proof of oral polio vaccination at least 4 weeks prior to travel.
Even though there are no general requirements, travelling to India without vaccinations is strongly discouraged. India presents several significant infectious disease risks, so the list of recommended vaccines is extensive:
- Hepatitis A: Highly recommended. India is considered a high-risk country for hepatitis A, as the virus spreads through contaminated food and water. Almost any traveller eating at local restaurants, markets, or street vendors is exposed. The vaccine (2 doses) prevents this acute hepatitis with over 95% effectiveness.
- Typhoid fever: Strongly recommended. The incidence of typhoid fever in the Indian subcontinent is high and, more concerning, there are antibiotic-resistant strains in India, Pakistan, Nepal, etc. Therefore, the typhoid vaccine is essential if you plan to travel through India, especially to rural areas or small cities where water hygiene is questionable. Even in major cities like Delhi or Mumbai, many travellers contract “enteric fevers” from contaminated food—so better to be vaccinated and cautious (always drink bottled water, etc.).
- Hepatitis B: India has an intermediate prevalence of hepatitis B, and given the possibility of requiring medical care or other exposures, it’s wise to travel protected. If you were born after 1990, you may already be vaccinated through childhood immunisation schedules; if not, it’s worth getting vaccinated.
- Tetanus-diphtheria: Essential to have up to date. In India, medical assistance might not always be readily available, and the risk of infected wounds is real—especially for adventurous travellers. Fortunately, most people are already vaccinated through routine immunisation.
- Rabies: Very important to consider. India reports the highest number of rabies deaths in the world annually; it's estimated that a large portion of global cases occur there, mainly from stray dog bites. The CDC classifies India as a high-risk country for rabies in travellers. Pre-exposure rabies vaccination is recommended if you’ll be staying >1 month or engaging in outdoor activities, cycling, motorbiking in rural areas where you may encounter animals. Also if you’re travelling with children (who tend to play with animals and may not report small bites). Getting vaccinated can save critical time if bitten, since rabies immunoglobulin is sometimes hard to find quickly in India. If unvaccinated, you must be extremely cautious to avoid contact with dogs, monkeys, cats, etc., and know where to find clinics that provide post-exposure treatment.
- Japanese Encephalitis: India is considered an endemic area for Japanese encephalitis in rural regions, particularly in the north and east (Uttar Pradesh, Bihar, Bengal, Assam...) during and after the monsoon season. The vaccine is recommended if you plan to live or spend extended time in rural agricultural areas of India, especially during the monsoon. For a typical tourist trip through major cities, Rajasthan, the Taj Mahal route, etc., it is generally not necessary. But if your itinerary includes rural villages or weeks travelling along the Ganges, consult your doctor.
- Poliomyelitis: India was declared polio-free in 2014, and travellers are no longer routinely vaccinated. However, due to proximity to Pakistan/Afghanistan (where the virus still circulates) and past imported cases, ensure your polio vaccination is complete. If you received your childhood doses but not an adult booster, you might consider an extra dose before travelling to very underdeveloped regions of India, as a precaution.

Do I need vaccinations to travel to Japan?
Japan is a destination with excellent health standards and few tropical diseases. There are no mandatory vaccinations for entering Japan (yellow fever or otherwise) - unless, of course, you are coming from a country with yellow fever, in which case many Asian countries apply the RSI regulations and may require yellow fever vaccinations.
In terms of recommended vaccinations, Japan does not require specific vaccine prophylaxis as do other countries. The health authorities basically advise you to have your routine vaccination schedule up to date, including tetanus-diphtheria, measles-rubella-mumps (MMR), as there have been outbreaks of measles in Japan in recent years among unvaccinated people, and seasonal flu if you are travelling in winter. If for some reason you didn't get polio or hepatitis B as a child in your home country, take the opportunity to get up to date, if only for general protection (not because Japan poses a particular risk of these infections).

What vaccinations are advisable for travel to Vietnam?
Vietnam has a similar health profile to Thailand: tropical climate, water- and mosquito-borne diseases, and developing hygiene standards. There are no mandatory vaccinations for entry (they would only require yellow fever if you're coming from an endemic area). But there are a number of recommended vaccinations.
Hepatitis A, Typhoid Fever, Hepatitis B, Tetanus-diphtheria, and assess Japanese Encephalitis and Rabies are advised for travel to Vietnam depending on the length and type of trip. This is due to similar reasons as in other neighbouring countries.

What vaccinations are required for travel to Mexico?
Mexico is a common travel destination where it's worth reviewing your vaccinations, even though it doesn’t impose specific requirements on tourists. There are no mandatory vaccines to enter Mexico for international travellers, except, once again, for yellow fever if you are coming from certain countries in South America or Africa. In that case, Mexico requires a yellow fever certificate for travellers over 1 year old arriving from at-risk areas.
For travel to Mexico, the recommended vaccinations mostly align with the standard immunization schedule, plus a few additional ones just in case: Hepatitis A, Typhoid, Tetanus, Hepatitis B, and possibly Rabies in certain situations.
- Hepatitis A: Although Mexico has made great strides in improving drinking water safety, there are still rural areas or small establishments where you could be exposed. The hepatitis A vaccine is highly advisable if you haven’t had it already, as the risk remains and prevention is easy and effective.
- Typhoid fever: Recommended if you plan to go beyond the more developed or touristic zones. For example, if you're trekking in Chiapas, eating at local eateries, or visiting remote villages, getting vaccinated against typhoid would be a smart precaution. In contrast, a resort holiday in Cancún might carry minimal risk — but many Mexico itineraries combine cities, archaeological sites, and rural areas, so better to be protected.
- Tetanus-diphtheria: This should be up to date (especially if you’ll be exploring jungles, cenotes, or engaging in activities where injuries are possible — this is basic protection).
- Hepatitis B: Recommended for frequent travellers or those staying for an extended period. Although Mexico doesn't have very high prevalence, there's still a chance of needing invasive medical care (e.g., in the case of a traffic accident). If you’re not vaccinated, it’s wise to get protected.
- Rabies: Mexico has reported few rabies cases in recent years (thanks to effective control campaigns), mainly in certain rural areas and among wildlife (especially bats). Rabies vaccination is not routinely recommended for the average tourist, unless you plan to engage in high-risk activities: caving with bats, volunteering in animal shelters, or long stays in rural regions far from medical facilities. In such cases, the pre-exposure rabies vaccine would be advisable.

What vaccinations do I need to have to travel to Egypt?
Egypt does not generally require vaccines for foreign tourists, with the exception of yellow fever: any traveller over 9 months of age arriving from a country with yellow fever risk must present an international vaccination certificate upon entry. This also applies to travellers who have spent more than 12 hours in transit through sub-Saharan Africa.
As for recommended vaccines for Egypt, they are similar to those for other warm-climate destinations with variable sanitary conditions: Hepatitis A, Typhoid fever, Tetanus, Hepatitis B, and ensuring that Polio and Measles vaccinations are up to date.
- Hepatitis A: Highly advisable. In Egypt, it's easy to get gastroenteritis from food or water, and hepatitis A is one of the viruses you could contract. It's best to be vaccinated and still take precautions with salads, ice, and untreated water.
- Typhoid fever: Recommended, especially if you plan to go off the typical tourist routes or eat at street stalls. In cities like Cairo, hygiene standards vary; and if you're visiting oases or rural areas along the Nile, the risk increases. Since typhoid can be severe, getting an oral or intramuscular vaccine before your trip to Egypt is a smart precaution.
- Tetanus-diphtheria: Essential (as for any trip). Think about potential cuts while climbing pyramids or temples, or minor accidents—desert environments are unforgiving if you're not protected against tetanus.
- Hepatitis B: Recommended for longer trips or if you might face risky situations. It's not mandatory, but being protected against hepatitis B is always a good idea anywhere in the world.
- Polio: Egypt has been polio-free for years, although it imported some cases from Pakistan in 2013 during the Syrian crisis. The WHO has since declared it polio-free again. Just make sure you completed your childhood polio vaccination. If so, no booster dose is generally needed for Egypt.
- Rabies: Rabies exists in dogs and cats mainly in southern rural areas of Egypt, but for the average tourist (visiting Cairo, Luxor, Aswan, or Red Sea resorts), exposure is nearly zero. Rabies vaccination is only advised for veterinarians, spelunkers, or those staying long-term in rural villages. For most travellers, it's not necessary.

What vaccinations are required for travel to Kenya?
Kenya, a top safari destination in East Africa, requires the yellow fever vaccine for certain travellers. Specifically, Kenya mandates a yellow fever vaccination certificate for travellers over 1 year old arriving from countries with a risk of the disease. This also applies to those who have spent more than 12 hours in transit through airports in such countries. Even if you're arriving directly (without transiting through West Africa, for example) and the vaccine is not technically mandatory, it is highly recommended if you are travelling to Kenya. There are rural border areas where the virus circulates sporadically, so for safety, the CDC and WHO recommend the yellow fever vaccine for virtually all travellers to Kenya aged 9 months and older (unless you're only visiting Nairobi and certain coastal areas).
As for other recommended vaccines for Kenya: Hepatitis A, Typhoid, Hepatitis B, Meningitis ACWY, Rabies, and Tetanus top the list, similar to the general recommendations for Africa. Let’s break them down:
- Hepatitis A: Strongly recommended; Kenya has a high risk of hepatitis A due to contaminated water and food. Any traveller sampling local cuisine—especially outside luxury lodges—should be vaccinated.
- Typhoid fever: Also very important. Outside Nairobi, water infrastructure is limited, and the risk of typhoid is significant. Get vaccinated if you plan to engage with local communities, eat at markets, or visit rural areas. Even on safari, you might pass through small towns with lower hygiene standards.
- Hepatitis B: Recommended for the same reasons as in other regions—not because transmission risk during the trip is high (it’s low with precautions), but because contracting it can have serious consequences, and the vaccine is highly effective.
- Meningococcal meningitis: Kenya sits at the southeastern edge of the African “meningitis belt.” Past outbreaks have occurred in northern Kenya. If you’re planning to volunteer, go on missions, or live in communities—especially during the dry season—it may be wise to get the ACWY meningococcal vaccine. It’s not generally recommended for casual tourists, but worth considering for long stays in areas like Turkana.
- Rabies: Rabies is present in Kenya’s wildlife (monkeys) and in dogs. Although safari-goers usually travel in vehicles and avoid direct contact with animals, the pre-exposure rabies vaccine is advised if you plan on adventurous ecotourism, camping, biking, or motorbiking through the country—where the likelihood of animal contact is higher and access to medical care might be limited. A nature photographer spending weeks on foot in reserves should seriously consider vaccination. For the typical safari tourist in a jeep, sleeping in lodges and only interacting with animals under guide supervision, the risk is low—but avoid touching animals, even if they seem tame.
- Tetanus-diphtheria: Definitely keep this up to date. Travel in Kenya may involve scrapes in the savannah, falls, or minor injuries, and hospitals may be far away—better to be immunized in advance.

Conclusion
Travelling informed and vaccinated is the best way to ensure your trip doesn’t come with unwanted health surprises. In this article, we’ve reviewed the mandatory vaccines (few, mostly yellow fever) and the recommended ones for several popular destinations.
Ultimately, travelling is an enriching experience, and health should not be an obstacle. With proper planning, vaccinations, and preventive measures, you can explore the world while minimizing risks. As you’ve seen, vaccines work by safely exposing your immune system to weakened pathogens or their fragments, training it to effectively defend against the real thing. Thanks to vaccines, today’s travellers no longer fear many of the diseases that once devastated expeditions. Take advantage of that progress: get informed, get vaccinated, and then, travel with peace of mind and enjoy.
Frequently Asked Questions (FAQ)
It depends on the destination. Some vaccines are mandatory (like yellow fever), others are recommended based on local health risks (hepatitis A, typhoid, rabies, etc.).
No mandatory vaccines, but hepatitis A and B, typhoid, tetanus, rabies, and Japanese encephalitis (for rural trips) are recommended.
Yellow fever (mandatory in many countries), hepatitis A and B, typhoid, tetanus, meningitis ACWY, and rabies. Malaria prophylaxis is also necessary in most regions.
Hepatitis A and B, typhoid, tetanus. Consider rabies and Japanese encephalitis if you're visiting rural areas or staying long-term.
Recommended vaccines include hepatitis A and B, typhoid, tetanus, polio, rabies, and Japanese encephalitis depending on your trip length and activities.
No mandatory vaccines. Keep your routine immunizations updated, including MMR, tetanus, and polio.
Hepatitis A and B, typhoid, tetanus are recommended. Also Japanese encephalitis (for rural stays) and rabies if in contact with animals.
Recommended: hepatitis A and B, typhoid, tetanus. Rabies only if engaging in high-risk activities like caving or rural stays.
Hepatitis A and B, typhoid, tetanus. Rabies only in very specific rural cases. Make sure polio and measles vaccines are up to date.
Yellow fever (highly recommended), hepatitis A and B, typhoid, tetanus, meningitis ACWY, rabies, and malaria prophylaxis.
Only in specific cases like yellow fever. Most are recommended based on destination-related health risks.
References
This guide was developed using official and specialized sources, including the World Health Organization (WHO) – International Travel and Health, the U.S. Centers for Disease Control and Prevention (CDC) – Traveler’s Health, Spain’s Ministry of Health – La Salud también viaja, and recommendations from the Spanish Vaccinology Association, among others. These institutions provide up-to-date, detailed information on international vaccination and are highly recommended as reliable sources for travellers who want to stay healthy while abroad.







